Does Mercury Conduct Electricity?

I bought this from my science shop yesterday so that I could perform more experiments.
Why is it a liquid?
The reason for mercury being a liquid is complex. It is heavy; a chunk of iron can float on mercury. Compared to other metals, it does not conduct heat well. However, it conducts electricity fairly well.
Mercury is the only metal that is a liquid at normal temperatures and pressure. What makes mercury so special? Basically, it’s because mercury is bad at sharing… electrons, that is.
Most metal atoms readily share valence electrons with other atoms. The electrons in a mercury atom are bound more tightly than usual to the nucleus. In fact, the s electrons are moving so fast and close to the nucleus they exhibit relativistic effects, behaving as if they were more massive than slower-moving electrons.
Why does it conduct electricity?
Mercury is a liquid metal. As with all metals, the outer electrons are detached from the nuclei and form a kind of “sea of electrons” in which the rest of the atoms sit. A small electrical force, in other words a voltage, placed across any two points in this sea will make the electrons move, and that constitutes a current and makes a metal electrically conducting.
I wanted to see that mercury can conduct electricity. I opened mercury bottle, it has an inner closure, I’m not going to take it out because we need it.

I’m going to put pins in it.

I will flip the bottle and the mercury will be in contact with the pins and the circuit will turn on.
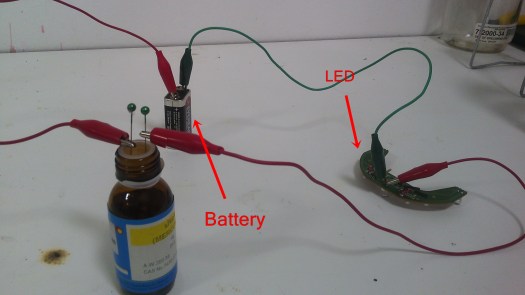
It works nicely!


This chemical will go into my favorite chemical list!
https://www.quora.com/Why-does-mercury-conduct-electricity-so-well
https://www.thoughtco.com/why-is-mercury-a-liquid-608454
















