How to Look at Pond Water With a Microscope
After the microscope slide series, I wanted real specimens from nature like pond water, which is what I’m doing today, and I have a pond in my backyard. I did this several times, it will be easy, so let’s get started.
Things you’ll need: Pond water, a jar, forceps eye dropper, microscope slides, cover slips, paper, and a microscope with 40x-100x magnification.
- Collect the water using the jar. I found 2 tadpoles and 1 mosquito larva in my collected water.

- Use the eye dropper to collect a small amount of the water from the jar.
- Place the microscope slide that you’re using on to a piece of paper
- Release one drop of the water onto the microscope slide from the eye dropper.

- Use forceps to carry the cover slip, then use it to cover the slide. This will spread the water out into a thin layer over the slide.
- Place the prepared slide into the microscope. Then, activate the microscope’s light.
I looked at the water under the microscope but I don’t see anything interesting. The only thing I see: dirt, string, and dots.
So I’m not going to look at the water. I wanted to see the organisms I collected.
I sucked the mosquito larva into the eyedropper.
Mosquito Larva:

I dropped the mosquito larva onto the slide. But I’m not going to put the cover slip on.
Yay! I can see it!


Look at it! Compare it to the one from the previous posts:
I wonder what will happen if I put the cover slip on.
And it appears that I crushed it…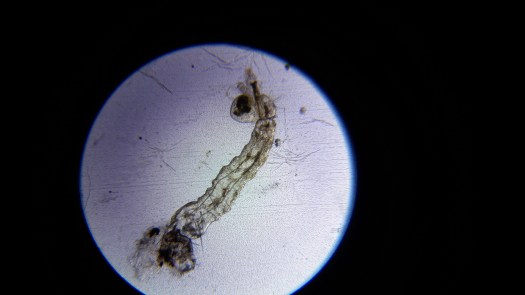
Let’s look at the tadpole:
Tadpole:
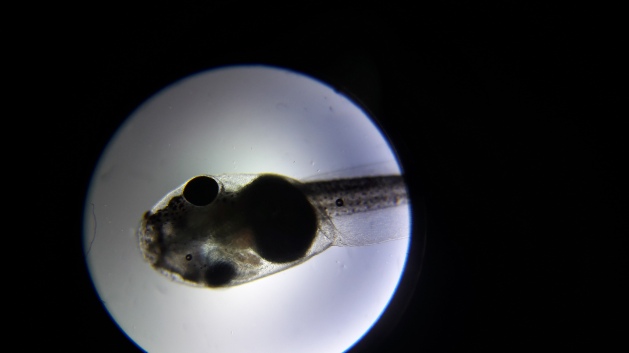
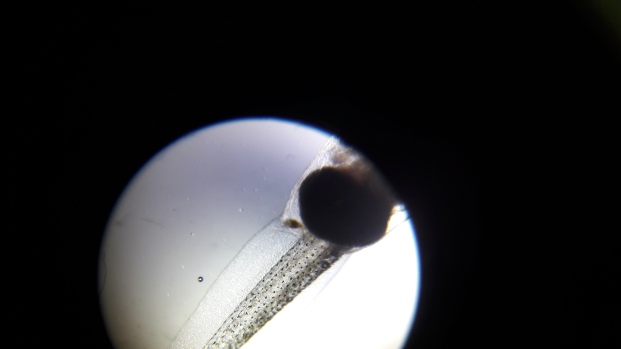
I put the cover slip on, and the tadpole crushed.
I released the left over tadpole back into the pond :D.
Do you like microscopes? Tell me in the comment section↓












